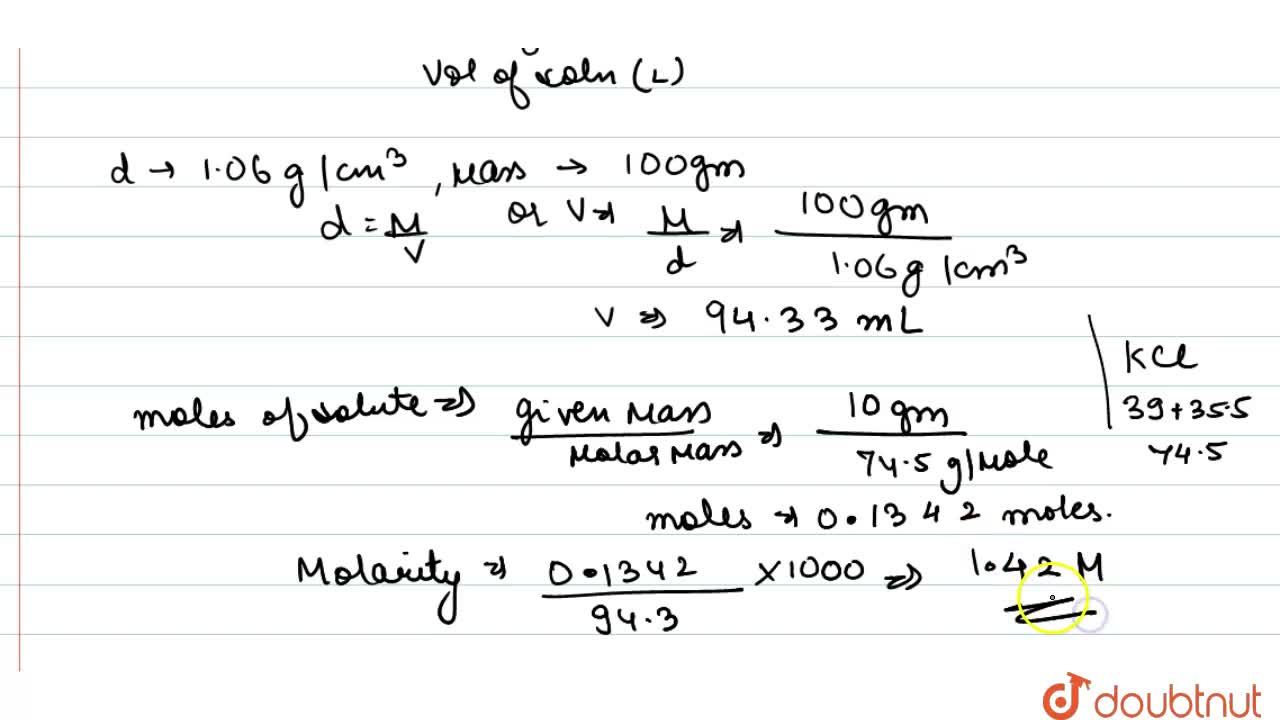
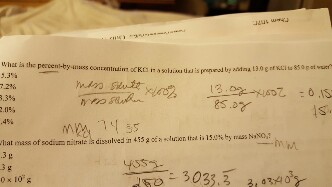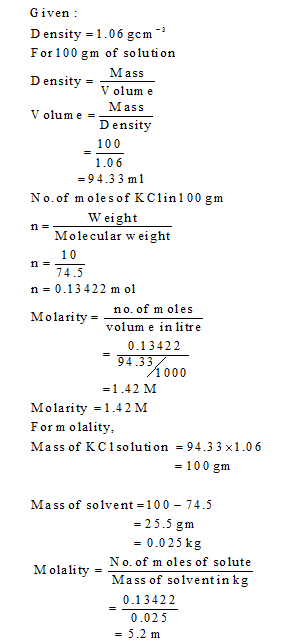The density of a 10.0% by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.
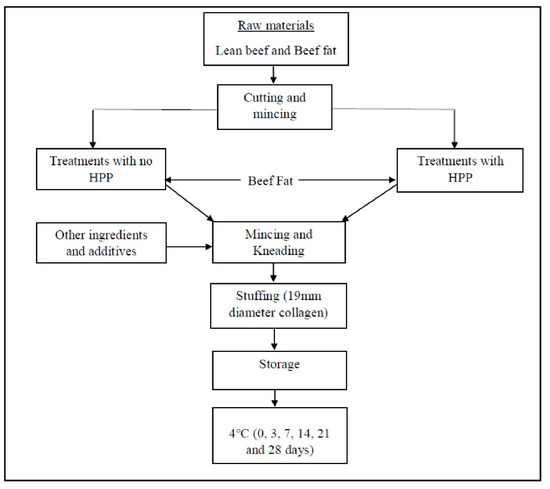
Processes | Free Full-Text | Effect of Partial Substitution of Sodium Chloride (NaCl) with Potassium Chloride (KCl) Coupled with High-Pressure Processing (HPP) on Physicochemical Properties and Volatile Compounds of Beef Sausage under

Aq KCL #solution of #density 1.2 g/ml has a #molality of 3.30 mol/kg. find #molarity. #jeemains2021 - YouTube
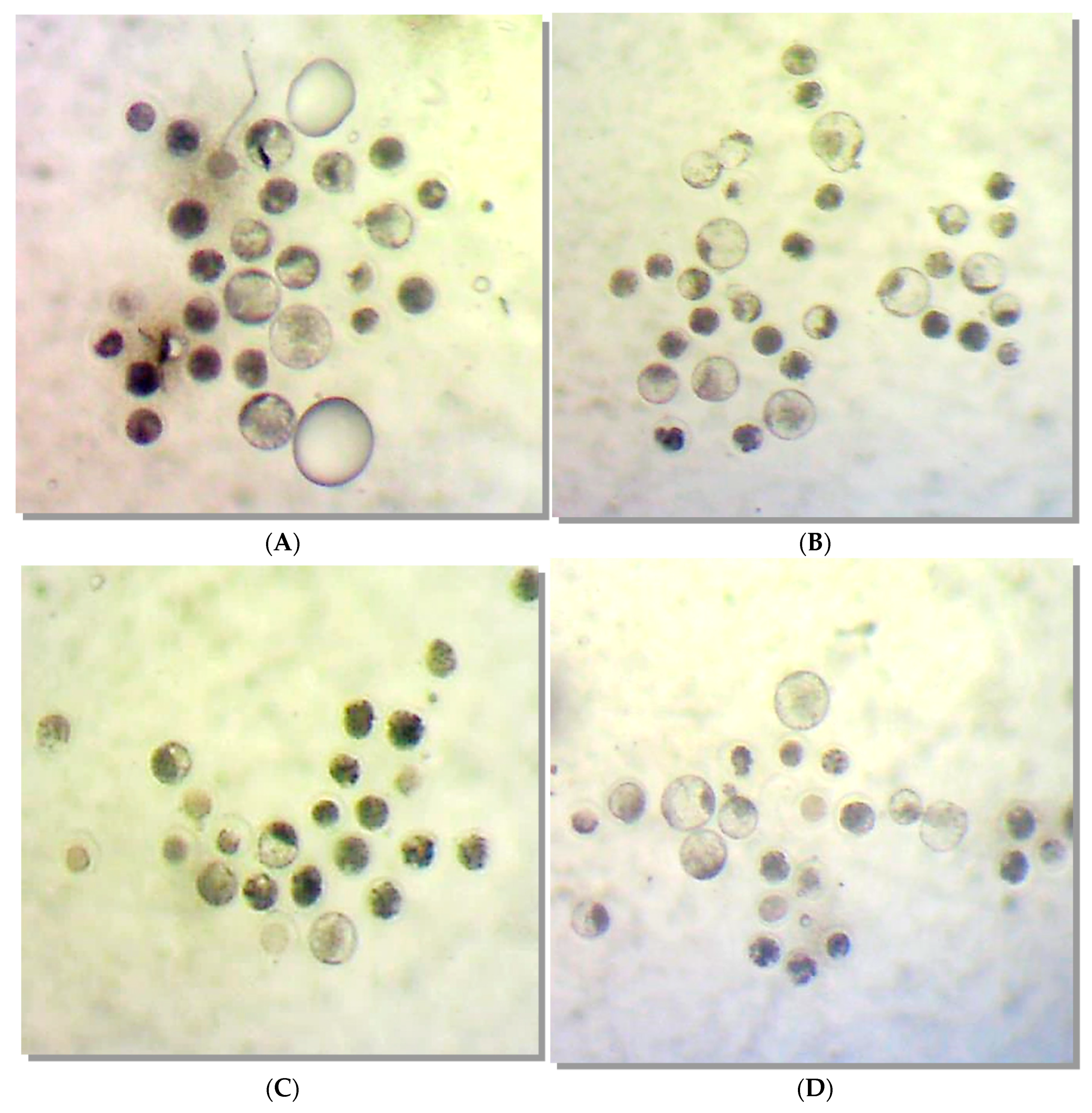
Molecules | Free Full-Text | Galectin-1 Used in Assisted Reproduction—Embryo Safety and Toxicology Studies
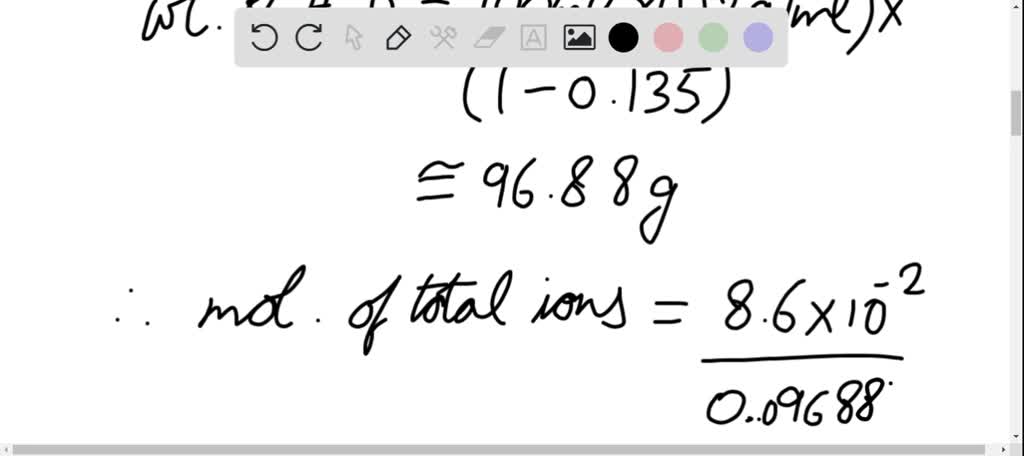
SOLVED:A 100.0 -mL aqueous sodium chloride solution is 13.5% NaCl by mass and has a density of 1.12 g / mL . What would you add (solute or solvent) and what mass

Quantification of Zeta-Potential and Electrokinetic Surface Charge Density for Colloidal Silica Nanoparticles Dependent on Type and Concentration of the Counterion: Probing the Outer Helmholtz Plane | The Journal of Physical Chemistry C
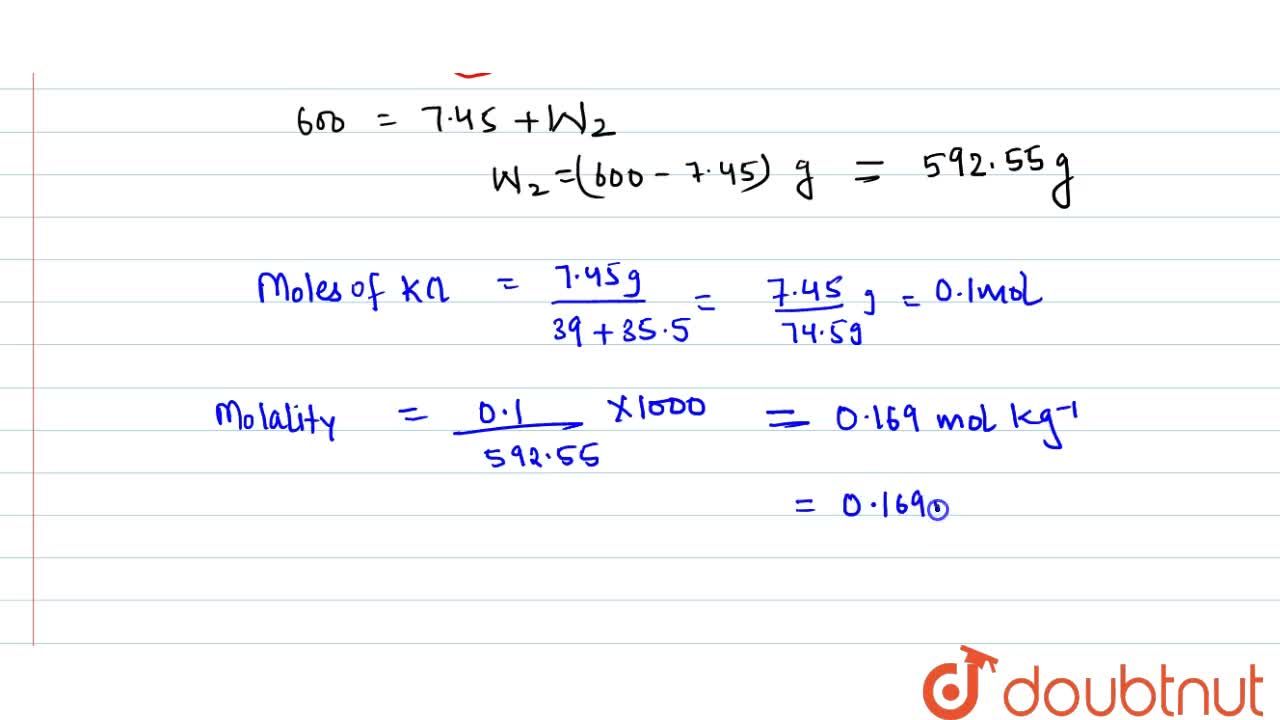
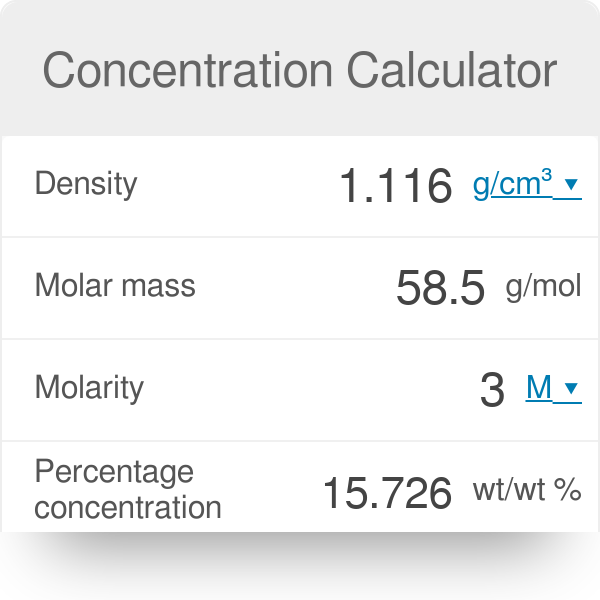
Calculate the molarity of KCl solution prepared by dissolving 7.45 g of KCl in 500 mL of the solution. (d(sol) = 1.2 g mL^(-1))

A solution is prepared by dissolving 4 g of NaOH to give 500 ml of it. Calculate the molality of the solution.

A solution of KCl has a density of 1.69 g mL^(-1) and is 67% by weight. Find the denisty of the solution if it is diluted so that the percentage by weight

The density of a 10.0% by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.

Measurements of Vapor Pressures of Aqueous Solutions in the NaCl–KCl–H2O System from 493.15 to 693.25 K in a Fused Silica Capillary High-Pressure Optical Cell | Journal of Chemical & Engineering Data
Change in the concentration of silicon-containing ions in the KCl melt... | Download Scientific Diagram
SOLVED: What is the Molar concentration of a solution with a volume 3.3 mL that contains 12 grams of ammonium sulfite? How many grams of copper (II) fluoride are needed to make

Small molecule SWELL1 complex induction improves glycemic control and nonalcoholic fatty liver disease in murine Type 2 diabetes | Nature Communications

An aqueous solution contains 30% w/v of urea , density of solution is 1.2 g/ml. Calculate mass of water in 100ml solution.

The density of a 10.0% by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.